I – Location of the thoracic aorta
The aorta has two segments, one in the rib cage, called the thoracic aorta, and another in the abdomen, called the abdominal aorta. The thorax and abdomen are separated by a horizontal muscle called the diaphragm. In the rib cage, the aorta looks like a walking cane with two parts going up and across like a “handle” (the ascending aorta and the arch of the aorta), and another segment going down (the descending aorta) that extends into the abdomen, passing through the diaphragm.
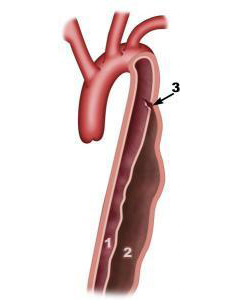
Figure 1: Thoracic and abdominal aorta
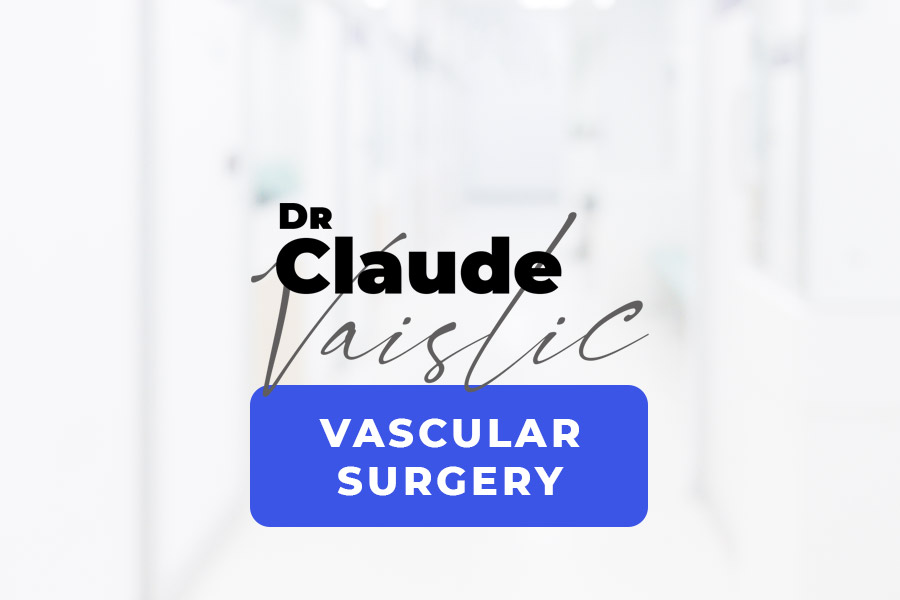
- 1. True lumen
- 2. False lumen
- 3. Entry tear
Four arteries branch off the aortic arch, supplying the arms (right and left subclavian arteries) and the brain (right and left common carotid arteries). On the right side of the body, the carotid artery and subclavian artery both arise from the aortic arch (brachiocephalic artery or trunk).
Figure 2: Aortic arch
II – Defining thoracic aortic aneurysm (TAA)
Normally, the aorta has an even diameter and parallel walls. The artery wall consists of three layers which are perfectly bonded together. The middle layer is made up of elastic tissue which acts like rubber. The aorta is under constant pressure due to the blood flow ejected from the heart. With each heartbeat, the aortic walls dilate and contract. Any anomaly of this elastic tissue weakens the arterial wall, which then tends to slowly form a bulge called an aneurysm. Once an aneurysm forms, it gradually grows, creating a vicious circle: the more the aorta dilates, the weaker its wall becomes and the faster it will continue to dilate.
An aneurysm can therefore be defined as a dilatation of the artery affecting all three layers of the arterial wall. It is most often spindle-shaped (or “fusiform”). More rarely, an aneurysm can be spherical (or “saccular”), like a bulge on an inner tube.
Aneurysms are classified by their location, shape, and cause. Thoracic aortic aneurysms (TAA) are located within the rib cage. Aneurysms can develop on the ascending aorta, aortic arch, or descending aorta. Additionally, one aneurysm may affect one or all of these sections. It can also extend onto the abdominal aorta, in which case it is called a thoracoabdominal aortic aneurysm (TAAA).
Thoracic aortic aneurysms occur much less frequently than abdominal aortic aneurysms. Aneurysms most commonly occur on the descending aorta and ascending aorta, and least frequently on the aortic arch.
III – Causes
Descending thoracic aortic aneurysms are most often caused by atherosclerosis secondary to plaque build-up on the artery wall. Atheromas occur more frequently in men over age 55. Other risk factors include smoking, high blood pressure, hyperlipidaemia (elevated blood cholesterol levels), and family history. Unlike abdominal aortic aneurysms, TAAs affect equal numbers of men and women. However, women are diagnosed on average ten years later than men (age 70 versus age 60).
TAAs may arise as a complication of an aortic dissection. During an aortic dissection, blood flows not only in the aorta but also between the layers of the artery wall, forcing the layers apart much like how we might separate the layers of an onion. The blood therefore circulates in two passageways: the aorta (true lumen) and the aortic wall (false lumen) (Figure 3). Dissection can substantially weaken the wall of the aorta, increasing the likelihood that it cannot withstand blood pressure and dilates to form an aneurysm. Thoracic aortic aneurysms can also be caused by conditions that weaken the layers of the aortic wall, particularly Marfan syndrome, a genetic disorder of the connective tissue. Ascending aortic aneurysms can be linked to aortic valve abnormalities.In rare cases, aneurysms may arise from infections.Aortic aneurysms can also be linked to rare inflammatory conditions affecting several tissues, such as Takayasu’s arteritis or giant-cell arteritis.Thoracic aortic aneurysms develop very slowly: 1 mm per year in the ascending aorta and 3 mm per year in the descending aorta. They start to develop more quickly after exceeding 40 mm in diameter. If aorticdissection occurs, they may develop even faster.
Figure 3: Aortic dissection
IV – Symptoms
Most thoracic aortic aneurysms (TAAs) produce no symptoms. Rather, they are discovered during a lung x-ray or a chest CT scan for another condition. If an aneurysm is found in the abdominal aorta or the popliteal artery of the knee, the patient is also checked for a thoracic aortic aneurysm. If a TAA does produce symptoms, however, these are due to its size or to complications. Patients may feel pain in their chest or between their shoulder blades, or they may have a cough or difficulty breathing. Sudden violent chest pain can indicate that the aneurysm is starting to rupture, requiring urgent emergency care.
V – Risks
In general, aneurysms do not produce any symptoms and will steadily grow. Once they reach a certain size, they may rupture, leading to internal haemorrhaging in the thorax (called hemothorax) or hemopericardium (provoking cardiac tamponade). If left untreated, the patient dies. The second risk, which also depends on the diameter of the aorta, is an occurrence of aortic dissection.
VI –Main exams and tests
Chest x-rays cannot be used for precise analyses of the aorta, but they do provide a picture of it and can help detect thoracic aortic aneurysms.
CT angiograms (CT scans with injection of iodine contrast medium) are the benchmark exam for analysing the wall of the aorta, measuring diameters, and precisely locating the aneurysm. Thanks to computer advances, 3D reconstructions are now possible. If a CT scan is out of the question (for example, if the patient is allergic to iodine), magnetic resonance angiography (MRA) can be performed with a non-iodine contrast material, gadolinium.
Cardiac ultrasound is helpful to analyse the heart, valves, and ascending aorta.
Endoscopic ultrasound: after local anaesthesia is administered to the mouth and throat, a probe is inserted into the oesophagus to image the heart and ascending and descending aorta. Arteriography involves making an incision in an artery under local anaesthesia, then inserting a catheter up to the aorta. This exam is now almost never required, given the quality of CT angiograms. It is sometimes performed to study the arteries that supply blood to the spinal cord.
VII – Main treatments
Surgical treatment will depend on the aneurysm’s cause, shape (fusiform or saccular) and diameter. Aneurysms caused by plaque build-up and smaller than 50 mm in diameter are regularly monitored. In all cases, it is crucial to control risk factors by quitting smoking, taking statins to lower cholesterol levels, and taking medication to lower blood pressure (particularly beta-blockers and ACE inhibitors). Patients with a genetic disorder of the connective tissue, such as Marfan syndrome, will be operated on earlier.
A – Open repair
To repair ascending aortic aneurysms, blood flow to the heart and ascending aorta must be interrupted and extra-corporeal circulation must be used. This means a pump takes over the heart’s functions and circulates blood while the heart and aorta are repaired. A vertical incision is made to divide the sternum (median sternotomy). The ascending aorta is then replaced by a vascular graft. Sometimes, during the same surgery, the aortic valve must be replaced and the coronary arteries (which supply blood to the heart) must be reimplanted.
For descending aortic aneurysms, an incision is made between the left ribs (thoracotomy). Although the heart does not have to be stopped, extra-corporeal circulation is often used to supply blood to the abdominal organs such as the liver, kidneys, and digestive tract while the aorta is being repaired. Aneurysms on the arch of the aorta are complex to treat. They require sternotomy and cardiopulmonary bypass.
B – Endovascular repair
This involves introducing an internal prosthesis (endograft) into the femoral artery using x-ray guidance until reaching the thoracic aorta. The endograft is loaded onto a stent and tightly folded into a sheath. Once the endograft is correctly positioned, the sheath is removed and the endograft opens up. This procedure requires “anatomical suitability”, i.e. a normal section of the aorta aboveand below the aneurysm for the endograft to be correctly anchored and sealed (Figure 4). The CT scan detects whether this suitability exists.
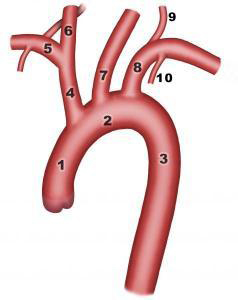
Figure 4 Aortic endograft
- 1. Ascending aorta
- 2. Transverse arch
- 3. Descending thoracic aorta
- 4. Brachiocephalic trunk
- 5. Right subclavian artery
- 6. Right common carotid artery
- 7. Left common carotid artery
- 8. Left subclavian artery
- 9. Left vertebral artery
- 10. Left internal thoracic artery
VIII – Risks of treatment
Ascending aortic repair carries a risk of potentially life-threatening complications such as stroke, heart attack, and infection of the sternotomy. The main risk of descending aortic surgery is paraplegia (paralysis of the lower limbs), since the arteries supplying blood to the spinal cord originate on the thoracic aorta. Respiratory problems, myocardial infarction, and organ failure (e.g. the kidneys and liver) can lead to death.
If anatomical suitability is adequate and an endograft is implanted in the descending aorta, postoperative mortality is lower, but there is still a risk of stroke or paraplegia. Endovascular repair makes it possible to treat patients presenting with high operating risk. It is a minimally-invasive procedure, but its long-term outcomes are not well-known. The procedure reduces blood loss and shortens the time spent both in intensive care and the hospital. If left untreated, aneurysms will continue to grow and the aortic wall will become increasingly weak, with a high risk of complication: dissection, rupture, or death.
IX – Medical follow-up
Follow-up involves controlling risk factors: keeping blood pressure and diabetes in check, lowering cholesterol levels with statins, and losing weight.It also includes lifestyle guidelines, such as quitting smoking, walking for 30 minutes three times a week, and getting five daily servings of fruits and vegetables.
Regular screening must also be done for other aneurysms (e.g. of the rest of the thoracic aorta, the abdominal aorta, or the popliteal arteries). Any changes to plaque build-up must also be checked (e.g. Doppler ultrasounds of the carotid arteries or monitoring the coronary arteries). Family members should also be screened for aneurysms and, in the case of a genetic disorder such as Marfan syndrome, should receive genetic counselling.
The operated area must be monitored with CT scans. The implantation of an endograft requires regular postoperative monitoring and follow-up for the rest of the patient’s life to verify that it stays in place and well-sealed.
Find out more…
I – Diameter as indication for surgery
Ascending aorta repair is recommended once its diameter reaches 55 mm, but may be indicated for smaller diameters if risk factors for complications are present, such as Marfan syndrome, family history of aortic dissection, or a bicuspid valve (one with only two leaflets; the aortic valve normally looks like a circle with three leaflets).
Descending aorta repair is recommended once its diameter reaches 65 mm, or 60 mm in case of Marfan syndrome.
Painful aneurysms must be quickly operated on, regardless of size. It is important to operate before dissection or rupture can occur. The critical diameters for the appearance of a complication are 60 mm for the ascending aorta and 70 mm for the descending aorta. In patients with no symptoms, the diameter of the aneurysm is the best basis on which to decide whether or not to operate.
II – Measuring the diameter of the aorta
The thoracic aorta displays complex geometry. Measurements are precise to within 4 mm. Echocardiography provides images of the first few centimetres of the ascending aorta, but may not detect aneurysms in its middle section. Transoesophageal echocardiograms may not provide images of the distal section of the ascending aorta. CT scans, on the other hand, generate images that look like horizontal “slices”. To precisely measure the diameter, an aortic median centreline must be reconstructed. The diameter is then measured perpendicularly to this line. The diameter of the aorta is not the same after the heart contracts (systole) or relaxes (diastole). It may also differ depending on whether or not the thickness of the aortic wall is taken into account. Ideally, then, to assess the growth of an aneurysm’s diameter, we need a CT scan synchronized with the heart, 3D reconstructions, and an identical measurement technique.
III – Choosing conventional open repair or endovascular repair
Just because endovascular treatment is usually simple to perform does not mean that we should change the indications for surgery and use it for small aneurysms. There are no scientific studies showing that the risk-benefit ratio favours early repair of small aneurysms. Recent studies provide figures on progress made in surgery of the descending thoracic aorta, with a reduction in mortality and complications, particularly paraplegia. Thoracic endovascular aneurysm repair (TEVAR) is theoretically advantageous for patients who are elderly or have other conditions that would contra-indicate conventional open repair or make it high risk. However, no studies have compared TEVAR to open repair in patients with high surgical risk. Finally TEVAR is not indicated for Marfan syndrome.
Studies with flawed methodology suggest that endovascular repair of thoracic aortic aneurysms reduces operative mortality and perhaps also complications such as paraplegia, respiratory problems, and kidney failure. Endovascular repair can require redo operations, generally also by endovascular means.
Given the lack of uncontested studies comparing open and endovascular repair, if the diameter of the aneurysm justifies repair, the choice of technique will depend on the patient’s age, any associated disease, the anatomy of the aneurysm, and patient preference.
IV – Reducing the risk of paraplegia
The risk of paraplegia depends largely on the extent of the aneurysm, with lower risk for thoracic aortic aneurysms than for thoracoabdominal aneurysms. For the latter, risk also depends on their extent. The intercostal arteries which help vascularise the spinal cord arise from the aorta, usually between the eighth thoracic vertebra (T8) and the second lumbar vertebra (L2).In open repair, several techniques can be considered to reduce the risk of paraplegia: pump perfusion of the lower body during clamping, drainage of the cerebrospinal fluid, reattachment of the intercostal arteries, and controlled hypothermia. When positioning an endograft covering T8-L2, collateral circulation must be maintained by preserving, if possible, the patency of the left subclavian artery and the hypogastric arteries. Paraplegia can occur if blood pressure drops. Although there are no clinical studies on drainage of the cerebrospinal fluid, this technique is often suggested.
V – Hybrid procedure
As with the abdominal aorta, the thoracic endograft must be able to anchor itself to the healthy zones of the normal aorta, above and below the aneurysm, and form a tight seal. In some cases, another artery, such as a carotid or subclavian artery, branches off this normal zone. In this case, a bypass is performed on arteries which cannot be sacrificed. The combination of open graft repair and endograft deployment is called the hybrid procedure. With the development of stent grafts with branches, the bypass procedure has become increasingly unnecessary.
VI – Marfan syndrome
Marfan syndrome is a rare genetic disease that causes some 5% of aortic dissection cases. In this syndrome, the tunica media weakens after its elastic fibres break down or its smooth muscle cells die. The protein known as fibrillin, encoded by a mutated FBN1 gene, is one of the components of the microfibrils that play a role in the formation of elastic fibres. Another consequence of Marfan syndrome is the deregulation of transforming growth factor beta (TGF-ß). Marfan syndrome is an autosomal dominant disease, which means that if either parent carries the genetic defect, there is a 50% chance of passing it on to any child. The gene that produces the syndrome if it mutates is found on chromosome 15. Several hundred mutations have been documented for this gene, all causing diseases of varying seriousness. Diagnosis is mainly clinical.
In this syndrome, the main cause of morbidity and mortality is linked to cardiovascular impairment. Prognosis is mainly based on the degree of damage to the aorta, particularly the risk of aortic dissection or rupture. The risk increases relative to the diameter of the aorta. In the past, before treatment for Marfan syndrome was available, life expectancy in sufferers was rarely more than 40 years. However, now they enjoy near normal life expectancy. Beta blockers slow the rate of aortic dilatation and may diminish the risk of complications. Losartan, an angiotensin II receptor antagonist, has been found effective in animal studies. The patient is advised to replace the aorta once the diameter of the ascending aorta reaches 50 mm.