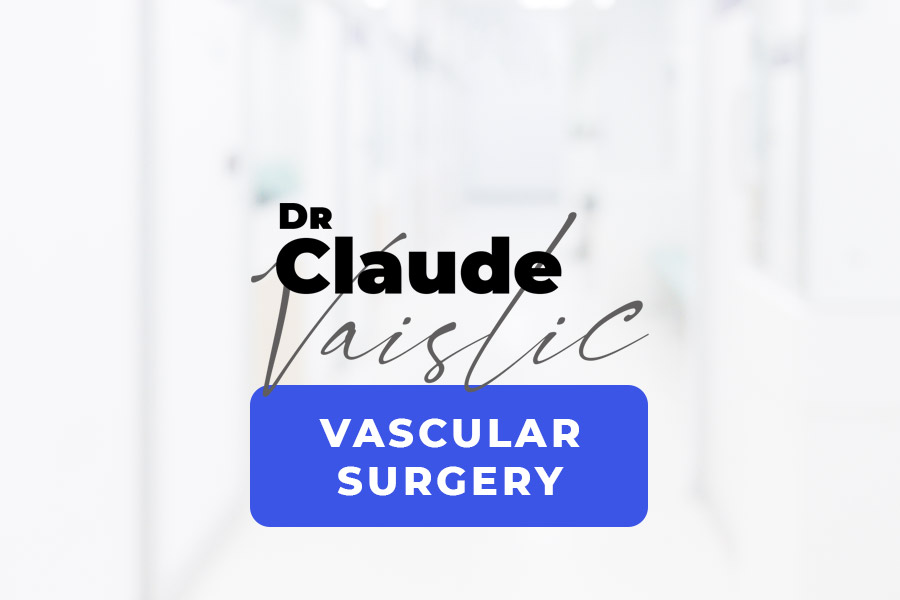
I – Defining thoracoabdominal aortic aneurysm (TAAA)
An aneurysm is an abnormal bulge in an artery that increases the artery’s diameter to over twice its normal size. Aneurysms can affect all arteries, but most often they are found on the aorta. The aorta is the largest vessel in the human body, and distributes blood to all the organs. It has four segments: (Figure 1)
- I – Ascending aorta, from the heart to the root of the vessels in the neck (i.e. supra-aortic vessels),
- II – Arch of the aorta, which gives rise to the vessels supplying the brain and arms (i.e. upper limbs),
- III – Descending thoracic aorta, from the aortic arch to the diaphragm,
- IV – Abdominal aorta, from the diaphragm to the point where the aorta bifurcates into the two iliac arteries (at the umbilicus).
Thoracoabdominal aortic aneurysms (TAAA) involve a simultaneous dilatation of both the thoracic and abdominal aorta. The arteries that carry blood to the kidneys, digestive organs (intestines, liver and stomach) and spinal cord branch off the thoracoabdominal aorta.
Figure 1: Anatomy of the aorta (segments I, II, III, IV)
II – Causes and most affected populations
Males over age 60 are the most affected, particularly if they present with cardiovascular risk factors such as smoking, high blood pressure, or a family history of cardiovascular disease. TAAAs constitute a rare condition (2.2 cases per 100,000). Atherosclerosis (hardening of the arteries) is one of the risk factors for developing aortic aneurysms. The artery walls are soft and compliant in young individuals. When the arteries harden, they become weak, making them unable to withstand blood pressure. Over time, they start to bulge. Some genetic disorders affecting the connective tissues, such as Marfan syndrome or Ehler-Danlos syndrome, can also be an underlying factor in some cases.
III – Symptoms
Thoracoabdominal aortic aneurysms (TAAAs) gradually develop over several years, and produce no symptoms in most cases. Most often, they are discovered accidentally during examination for another complaint.
Sometimes a pulsatile abdominal mass is palpated. If the aneurysm ruptures, the patient may experience abdominal or back pain, dizziness, or fainting due to arterial hypotension (low blood pressure), or they may go into shock from internal haemorrhage.
IV – Risks
The main risk of a TAAA is rupture of the aneurysm, leading to massive internal haemorrhage. This complication causes death is over 90% of cases.
V – Main exams
Aortic aneurysms are most often discovered during the following examinations for other diseases:
- Abdominal ultrasound, in which ultrasound waves are transmitted through the tissues and reflected by the body’s structures to produce an image.
- CT scan, which uses x-rays. The body’s tissue structures block x-rays to varying degrees. Sensors can therefore detect the intensity of the x-rays after exiting the body and generate images that look like “slices” of specific areas.
- Magnetic resonance imaging (MRI) uses magnetic waves, not x-rays. The body’s various tissues produce a signal in response to the MRI wave. This signal is then used to computer generate an image.
CT scans and MRIs establish the aneurysm’s size and location, and therefore help determine whether treatment is necessary.
VI – Main treatments and their risks
The goal of TAAA treatment is to prevent rupture. The decision to operate on a TAAA depends on its size (> 6 cm), rate of growth (> 0.5 cm over 6 months), and location, as well as the patient’s overall health. If the aneurysm is larger than 6 cm and/or rapidly expanding, your surgeon will weigh the benefits of treatment against the risks. Thoracoabdominal aneurysms are the most complicated aneurysms to repair, given that this segment of the aorta gives rise to the arteries supplying blood to the digestive system, kidneys, and spinal cord.
There are three surgical techniques for treating TAAAs: conventional surgery known as open repair (OR), endovascular aortic repair (EVAR), and a hybrid procedure.
- Open repair (OR): the thorax and abdomen are opened to access the thoracoabdominal aorta. After the aorta is clamped, the aneurysm is replaced with a patch sewn to the healthy aorta on either end of the aneurysm. The visceral arteries (i.e. the celiac trunk and superior mesenteric artery) and the renal arteries are anastomosed (attached) to the visceral aortic patch (VAP). Extra-corporeal circulation may also be needed to continue to pump oxygenated blood to the lower extremities during open TAAA repair.
- Endovascular aortic repair (EVAR) involves placing a stent graft inside the aneurysm without opening the thorax or abdomen. The stent graft is introduced through the femoral arteries via an incision in the groin. The stent graft can also be called an endograft. It has holes (known as “fenestrations”) and/or branches to be placed in the visceral and renal arteries to maintain blood flow (Figure 2). You could think of the stent graft as a synthetic aorta placed inside the diseased aorta. The diseased aorta has no more contact with the circulating blood, and therefore no longer risks rupturing
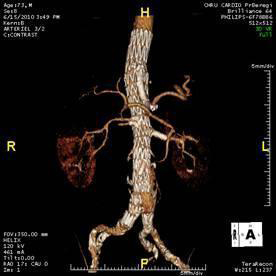
Figure 2: Fenestrated endovascular repair (FEVAR) for a type III TAAA, with one branch for the celiac artery and fenestrations for the superior mesenteric artery and renal arteries.
- The hybrid procedure combines both these methods. First, a bypass graft is attached to the visceral and renal arteries through conventional open repair via an incision in the abdomen.Then, non-fenestrated stent grafts are deployed to exclude the aneurysm sac.)
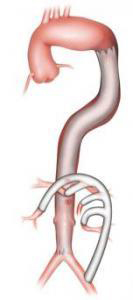
Figure 3: Hybrid endovascular procedure featuring stent graft exclusion of the TAAA and extra-anatomic bypass to the visceral arteries
Whichever technique is used, the following serious complications may arise after the operation:
- Paraplegia, i.e. paralysis in both legs (lower extremities) caused by insufficient blood flow to the spinal cord (spinal cord ischemia),
- Kidney failure (possibly requiring dialysis) caused by insufficient blood flow to the renal arteries,
- Visceral ischemia (possibly requiring removal of a portion of the intestines) caused by insufficient blood flow to the visceral arteries,
- Death due to multiple organ failure.
Endoleaks – leaks outside of the endograft into the aneurysm sac – may be found after endovascular repair. If these leaks are abundant, they require additional management. However in most cases, they are slow and resolve spontaneously during follow-up.
Find out more…
After surgery, you will be hospitalized for about ten days, if there are no complications. A six-week to three-month convalescence period is required.
A follow-up CT scan and Doppler ultrasound are performed six months after surgery, and then once every subsequent year.
The most widespread system in use, the Crawford classification, describes four types of TAAA (Figure 4). Type I extends from the beginning of the left subclavian artery to the renal arteries. Type II
encompasses the entire descending and abdominal aorta. Type III encompasses the thoracic aorta from the sixth rib to the bifurcation of the aorta. Finally, type IV extends from the diaphragm to the bifurcation of the aorta. Both the location and extent of the aneurysm on the thoracoabdominal aorta affect treatment outcomes (type IV has the best outcomes).
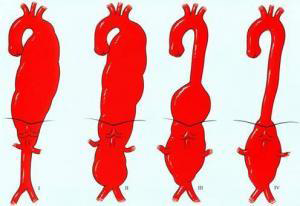
Figure 4: Crawford classification of TAAAs
TAAAs have a low incidence rate. Indications for surgery include the size of the aneurysm and/or its clinical and pathological course, with surgery generally being recommended once the maximum diameter exceeds 6 cm. If a connective tissue disorder is diagnosed, such as Marfan or Ehler-Danlos syndrome, surgery is recommended for smaller diameters, given the high risk of dissection and rupture. The conventional approach – open repair replacing the diseased portion of the aorta with a patch – has good outcomes in terms of patency but at the price of high perioperative mortality. Since 2001, several teams have managed these TAAAs through entirely endovascular means, thanks to the new generation of fenestrated multi-branch endografts.
The choice between open or endovascular repair depends on the analysis of the aneurysm’s morphology and the patient’s physiological condition. Perioperative morbidity and mortality are probably lower with EVAR, but long-term outcomes are still being studied. This new technique cannot be used to treat all TAAAs. The tortuosity of the aorta and the iliac arteries and the quality of the aortic wall (e.g. mural thrombus, calcification) can be contraindications for EVAR.
The endograft is made of a self-expanding metallic stent covered with a polyester material (Dacron®) to form an impermeable tube. It is preloaded on a delivery catheter (stent delivery system). In most cases of EVAR, several modular extensions are overlapped to achieve total exclusion of the TAAA. If the endograft covers the orifice of a visceral artery, blood supply is selectively ensured through fenestrations or branches in the endograft. A covered stent tightly bridges the endograft fenestration or branch and the trunk of the visceral artery to prevent leaks.
Mortality and spinal cord ischemia are the most feared post-operative complications. The other most common complications are kidney failure, stroke, congestive heart failure, and respiratory failure. Measures must be taken to protect the spinal cord in any, or a combination of, the following cases: an extended TAAA (classes I to III; prior aortic surgery; and occlusion of the left subclavian artery and/or hypogastric arteries. All of these situations increase the likelihood of acute spinal cord ischemia. To mitigate this, some teams offer CSF drainage.